In conversation with Jayadeep Unni, Founder of Sensivision Health
(December 2022)
Interviewed by N. Ramakrishnan

“Whatever it takes, sticking with the core till you see success is the most important attribute that a BIG grantee or a BIG applicant should have,” says Jayadeep Unni, Founder, Sensivision Health Technologies Pvt. Ltd., a Bengaluru-based venture that got funds under the BIG (Biotechnology Ignition Grant) scheme in 2015. He adds that as far as start-ups in the medical devices space are concerned, to succeed one must have tenacity and perseverance to see through the ups and downs. Funding is just one aspect of the journey, albeit an important one.
According to Unni, founders applying for assistance under BIG should have clarity on what they are doing, the idea and the solution, working with the stakeholders so that there is no confusion at a later stage on the product. It is better to resolve early in the journey questions over the product, viability and the business. The BIG panel does its best, but there have been instances where, either due to lack of guidance or lack of proper understanding, the project did not take off even after obtaining assistance under the BIG scheme.
It is here, he points out, that it is extremely important for founders and their ventures to identify a guide and a mentor early on in their journey. Sensivision, he adds, was fortunate to get help from the Pune-based technology incubator Venture Center and mentorship from its director, Dr. V. Premnath, right through the BIG process and even later, when Venture Center came in as an equity investor along with C-CAMP (Centre for Cellular and Molecular Platforms).
Sensivision is a medical devices company working in the neo-natal space. It has developed a product that helps in diagnosing and treating hypoxic ischemic encephalopathy in new-borns, a complication caused by birth asphyxia. Unni, who has a Bachelor’s in Biomedical Engineering from Cochin University and a Master’s in that subject from New Jersey Institute of Technology, worked in companies in the medical devices space in the US, obtained an MBA in Entrepreneurship and Finance from the University of Santa Clara, before returning to India in mid-2013. He was part of the Stanford Biodesign program, where he wanted to understand problems in the healthcare space that needed to be solved. He spent time understanding issues thanks to the program, where he worked with doctors and nurses in a few private and district hospitals in Karnataka. It was during this program that he narrowed down his area of
interest to the neo-natal domain.
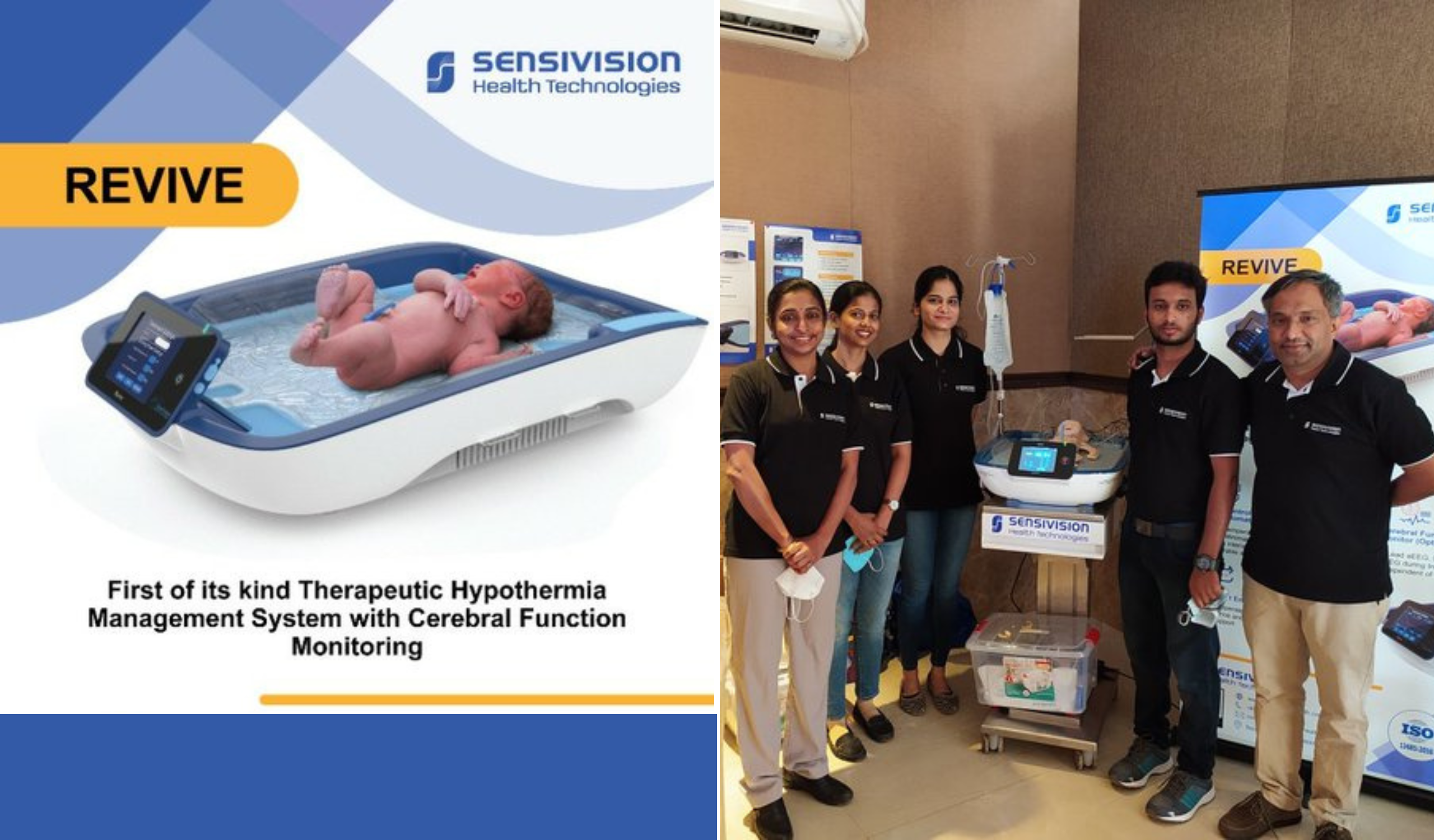
The problem, says Unni, of hypoxic ischemic encephalopathy (HIE) is alarming in India and most often babies getting into HIE were not being treated. The babies either died or suffered brain damage. The device available in the market to treat it had to be imported and was expensive. This was the problem that Sensivision sought to solve when it was founded in July 2015. The company had developed a table-top engineering concept and the BIG scheme helped it develop a prototype that could go through engineering standard testing. “Venture Center being our partner, especially Dr. Premnath, was a bedrock. Where we are today is because of him. A lot of motivation, a lot of timely support helped us navigate through the medical devices field, especially getting various clearances,” says Unni. Sensivision, according to him, has raised close to ₹2.5-3 crores so far to take its product to the market, the bulk of it coming through grants.
The company launched its product, called Revive, in early 2022. It is deployed in a few hospitals in various parts of India in neo-natal ICUs. The device is called a servo-controlled device where the baby’s core body temperature is closely monitored and based on the temperature, the device will take a decision on either to cool it or warm it. It is the only device, says Unni, that does both diagnosis and treatment. The 46-year-old Unni explains that in the case of babies in danger of slipping into HIE, the body temperature needs to be brought down from a normal of around 37 degrees Celsius to about 33.5 degrees Celsius. The fully automated device has a polymer blanket through which a fluid is circulated, which is used to monitor and regulate the body temperature. The treatment is called therapeutic hypothermia.
The Pune-based deep tech incubator Venture Center is one of the partners managing the BIG Scheme, which is a flagship program of BIRAC (Biotechnology Industry Research Assistance Council), a public sector enterprise set up by the Department of Biotechnology of the Government of India. The scheme is the largest early-stage biotech funding program in the country and provides grant up to ₹50 lakhs over an 18-month period based on milestones being achieved. The BIG Scheme aims to encourage more start-ups in the biotech space, helping them upscale and validate proof of concept. Sensivision, according to Unni, has got ISO13485 certification for the facility where it makes the device. It can make up to 50 devices a month. Unni anticipates demand going up as awareness about the product and the treatment increases.
![]()
